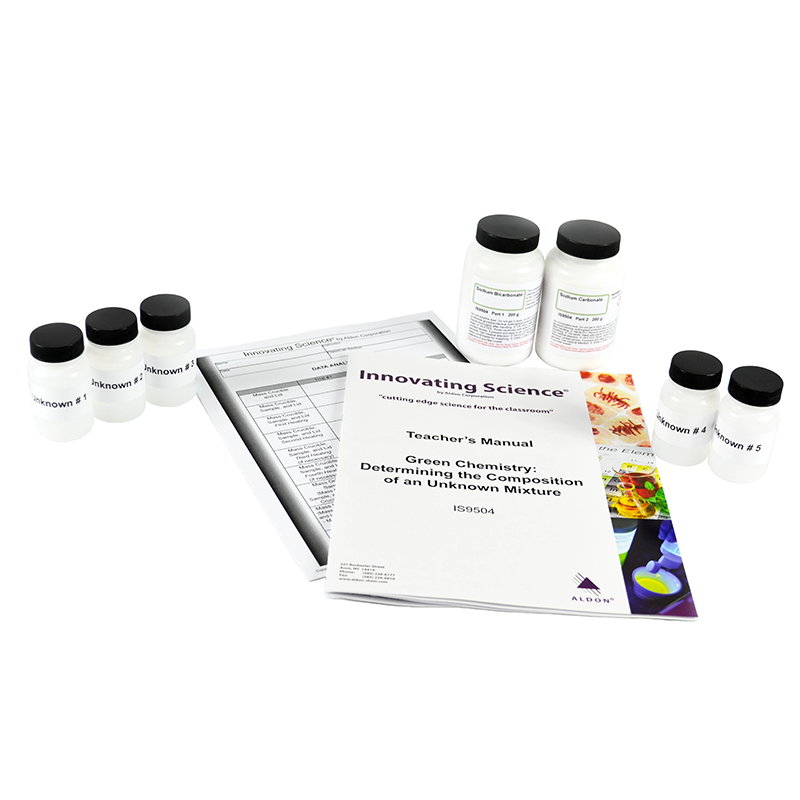


Often times, the composition of a mixture may contain a variety of unknown components. In some cases, the components of a mixture may be known but the exact amount of those components in the mixture is not. Analytical chemists often have a variety of tools and techniques to analyze unknown substances and arrive at conclusions with regards to the compounds in the mixture. In this activity, students will determine the percent composition of sodium carbonate and sodium bicarbonate in an unknown sample. The mixture is heated vigorously until the sodium bicarbonate is completely decomposed to sodium carbonate. The only other products of the reaction are carbon dioxide and water. After performing the necessary calculations, students will determine the percentage of sodium bicarbonate that was present in their original sample.
Resources

